Watch this on Rumble: https://rumble.com/v6lcxm7-lipid-nanoparticles.html
Hydrogels are water-based, soft materials that have become a cornerstone in biomedical research and applications. Their unique properties—such as high water content, flexibility, and biocompatibility—make them ideal for a wide range of uses, including drug delivery, tissue engineering, and regenerative medicine. Hydrogels are three-dimensional networks of polymers that can absorb and retain large amounts of water. These polymers are crosslinked, meaning they form a stable structure that doesn’t dissolve in water. This structure allows hydrogels to mimic the natural environment of human tissues, making them highly compatible with biological systems.
One of the key properties of hydrogels is their high water content, which can reach up to 90%. This creates a moist environment that supports cell growth and tissue repair. Additionally, hydrogels are biocompatible, meaning they are non-toxic and interact well with living tissues, reducing the risk of adverse reactions. Scientists can also tune the mechanical strength, porosity, and degradation rate of hydrogels to suit specific applications. Some hydrogels are even “smart” materials, capable of responding to external stimuli like temperature, pH, or light, which makes them particularly useful for targeted therapies.
Hydrogels have a wide range of applications in the biomedical field. In drug delivery, they are used to control the release of drugs over time. Their porous structure allows drugs to diffuse slowly, ensuring sustained and targeted delivery, which is especially beneficial for treating chronic conditions like diabetes or cancer. In tissue engineering, hydrogels serve as scaffolds for growing new tissues. They provide a supportive environment for cells to attach, multiply, and form functional tissues, such as cartilage, bone, and even heart tissue. Hydrogels are also excellent for wound healing, as they keep the wound moist, promote healing, and can deliver antimicrobial agents directly to the site. Their flexibility and comfort make them ideal for dressing burns or surgical wounds. In regenerative medicine, hydrogels are used to repair damaged tissues and organs by releasing growth factors or stem cells that stimulate the body’s natural healing processes.
Recent advancements in hydrogel technology have further expanded their potential. Self-healing hydrogels, for example, can repair themselves after damage, extending their lifespan and reliability. Injectable hydrogels can be injected into the body in liquid form and then solidify at the target site, making them minimally invasive. Nanocomposite hydrogels, which incorporate nanoparticles, offer enhanced mechanical properties and additional functionalities, such as conductivity or antimicrobial activity. These innovations are pushing the boundaries of what hydrogels can achieve in medicine and beyond.
Despite their immense promise, there are still challenges to overcome with hydrogels. Controlling their degradation rate to match specific applications is one such challenge. Scaling up production without compromising quality is another. Additionally, improving how hydrogels interact with the body to avoid immune responses or rejection is critical for their long-term success. Future research aims to address these challenges and develop even more advanced hydrogels. For example, combining hydrogels with other technologies, like 3D printing, could revolutionize personalized medicine and on-demand tissue fabrication.
Hydrogels are a versatile and powerful tool in modern medicine. From delivering life-saving drugs to regenerating damaged tissues, their potential is vast. As research continues to push the boundaries of what hydrogels can do, they are poised to play an even greater role in improving healthcare outcomes worldwide. Their ability to mimic natural tissues, respond to stimuli, and be tailored for specific needs makes them an invaluable resource in the ongoing quest to advance medical science and patient care.
Hydrogel technology and related biomedical advancements, including drug delivery systems and tissue engineering, are being studied and applied in various regions, including Africa. The extent and focus of these studies often depend on local healthcare needs, funding, and research infrastructure. Africa faces a high burden of infectious diseases such as malaria, HIV/AIDS, and tuberculosis, making hydrogel-based drug delivery systems particularly relevant. For example, hydrogels are being investigated as potential carriers for controlled drug delivery to improve treatment outcomes for malaria by delivering antimalarial drugs in a sustained manner, which could reduce the frequency of doses and improve patient compliance. Similarly, research is exploring hydrogels for delivering antiretroviral drugs for HIV/AIDS, potentially through injectable or implantable systems, which could revolutionize treatment in regions with limited healthcare access.
In addition to infectious diseases, hydrogels are being studied for their ability to promote wound healing and treat burns, which are common in many African countries. Access to advanced wound care is often limited, and hydrogels offer a promising solution due to their ability to maintain a moist environment, deliver antimicrobial agents, and reduce infection. This makes them particularly valuable in low-resource settings where traditional wound care options may be scarce. Furthermore, hydrogels are being explored for regenerative medicine and tissue engineering, which could have significant applications in Africa. For instance, hydrogels could be used to regenerate skin tissue for burn victims, who often face limited treatment options, or to repair bone and cartilage for patients with injuries or degenerative conditions.
Hydrogels are also being researched as delivery systems for vaccines, which could have significant implications for public health initiatives in Africa. For example, hydrogels could be used to deliver vaccines in a single dose that releases over time, reducing the need for multiple injections and improving vaccination coverage in remote areas. Additionally, hydrogels could help stabilize vaccines, making them easier to transport and store in regions with limited refrigeration, which is a common challenge in many parts of Africa. These advancements could play a critical role in improving vaccination rates and combating preventable diseases.
Some African institutions and researchers are actively exploring hydrogel technology, particularly in countries like South Africa, where universities and research centers are at the forefront of biomedical research on the continent. These institutions are conducting studies on hydrogels for drug delivery and tissue engineering, often in collaboration with international partners. Such collaborations are essential for developing and testing hydrogel-based solutions tailored to local healthcare challenges. However, despite the potential benefits, there are significant challenges to the widespread adoption of hydrogel technology in Africa. Limited funding for research and development can hinder progress, and many regions lack the infrastructure needed to manufacture and distribute advanced biomedical materials. Additionally, there is a need for greater awareness and training in the use of advanced materials like hydrogels among healthcare providers to ensure their effective implementation.
Hydrogel technology is being studied and applied in Africa, particularly in areas like drug delivery, wound care, and regenerative medicine. While challenges such as funding, infrastructure, and training remain, the potential benefits are significant, especially for addressing infectious diseases, improving wound care, and advancing public health initiatives. As research and infrastructure continue to grow, hydrogels could play a transformative role in improving healthcare outcomes across the continent. By addressing these challenges and leveraging local and international collaborations, Africa can harness the potential of hydrogel technology to meet its unique healthcare needs.
In the year 2000, Children’s Hospital of Philadelphia (CHOP) was granted Patent US6333194B1, titled Hydrogel Compositions for Controlled Delivery of Virus Vectors and Methods of Use Thereof. A viral vector is a modified virus designed to deliver genetic material into cells, commonly used in gene therapy and vaccines. These vectors are rendered harmless by removing the disease-causing components, though they still require careful handling due to their ability to deliver genetic material. The patent describes a method for encapsulating viral vectors within nano-sized hydrogels, enabling delivery into cells via mRNA gene therapy.
Hydrogels are soft, water-based materials capable of expanding and retaining water. Composed of crosslinked polymers, they form a 3D structure that absorbs water without dissolving. Hydrogels have diverse applications, including tissue engineering, drug delivery, and regenerative medicine. The duration a drug remains in a hydrogel depends on its design and the drug itself, ranging from hours to months, with some engineered for sustained release over a year or more. This is achieved through controlled diffusion and degradation mechanisms.
In 2004, US Patent US20060251719A1, Sustained-Release Hydrogel Preparation, was granted, focusing on controlling the timing of drug release within hydrogels. These patents, though dated, have likely been refined by 2019.
Carrie Madej, a physician with a deep commitment to humanity and holistic wellness, emphasizes the sacredness of life and the importance of balancing body, mind, and spirit. She advocates for physicians to educate patients on health and identify root causes of disease. Graduating from Kansas City University of Medical Biosciences in 2001, she now educates on vaccines, nanotechnology, and human rights. Despite being heavily suppressed, her message resonates with many. She warns that 75% of the global population has received COVID-19 mRNA gene therapy, potentially carrying Marburg virus, a situation she describes as a crime against humanity.
Hydrogel nanolipid particles, combining hydrogels and nanoparticles, are used in drug delivery and tissue engineering. Patent US9012240B2, granted in 2009, describes porous hydrogel nanoparticles that sequester analytes for immobilization, using an open polymeric meshwork to enclose affinity bait.
Bill Gates has been criticized for promoting mRNA vaccines in Africa, with claims that these are not traditional vaccines but experimental gene therapies. Gates’ foundation has invested $40 million in mRNA vaccine production in Africa, aiming to address vaccine inequities. Critics argue that African nations, which were less vaccinated during COVID-19, had lower per capita deaths, raising questions about the efficacy of these vaccines. Gates’ initiatives have sparked concerns about depopulation and the use of Africans as test subjects.
Dr. Dave Martin highlighted that the National Institute of Allergy and Infectious Diseases (NIAID), led by Dr. Anthony Fauci, funded lipid nanoparticle (LNP) technology used in Moderna’s and Pfizer-BioNTech’s mRNA vaccines. LNPs are synthetic lipids that encapsulate mRNA, forming nanoparticles. U.S. Patent 8,999,351, issued to Tekmira Pharmaceuticals, was supported by an NIAID grant, though the funds were initially awarded to Alnylam Pharmaceuticals. Legal disputes over trade secrets between these companies further complicate the narrative.
The number of LNPs or viral vectors in a single vaccine dose is staggering: AstraZeneca’s vaccine contains 50 billion viral vectors, Pfizer-BioNTech’s has ~40 billion LNPs, and Moderna’s contains ~10 billion LNPs. Each LNP can produce 1,000 spike proteins, potentially resulting in trillions of pathogenic spike proteins in the body. This raises concerns about the long-term effects of these nanoparticles, which can migrate from the injection site to other parts of the body, including vital organs and the bloodstream.
The potential for LNPs to cause adverse effects is significant. If LNPs escape the muscle tissue, they can transfect cells in blood vessels, organs, and lymph nodes, leading to inflammation, clotting, and cell death. This has been linked to myocarditis, thrombosis, and other severe conditions. The aspiration technique, recommended in Denmark, reduces but does not eliminate these risks.
Bill Gates’ involvement in vaccine development has drawn criticism, particularly given his family’s history in eugenics. His comments about “messing around” with lipid nanoparticles and the ease of producing mRNA have raised alarms. Critics argue that Gates’ focus on population control and profit-driven initiatives pose significant risks to public health.
The United Nations has acknowledged that a polio vaccine initiative funded by Gates has caused outbreaks of vaccine-derived polio in Africa. Similar incidents have occurred in Pakistan, Afghanistan, and Iran. These failures highlight the risks of using live virus vaccines, which can mutate and cause new outbreaks. Gates’ continued involvement in vaccine development, including COVID-19 vaccines, has sparked widespread concern.
The development and deployment of mRNA vaccines and hydrogel-based delivery systems represent significant advancements in biotechnology. However, the potential risks, including adverse effects and unintended consequences, cannot be ignored. The involvement of figures like Bill Gates and the complex web of funding and patents raise ethical and safety concerns that demand rigorous scrutiny. As the world navigates the challenges of modern medicine, it is crucial to prioritize transparency, safety, and the well-being of all individuals.
Can these amazing discoveries be abused? Absolutely as we have learned from the Bill & Melinda Gates foundation and their supposed depopulation agenda. The foundation has faced criticism and legal challenges related to its funding of vaccine programs, particularly in developing countries. Some critics have alleged that these programs have caused harm or unintended consequences, though these claims are often highly contentious and not always substantiated in court. The foundation has also been involved in disputes over patents and intellectual property, particularly in the context of its funding for medical research and vaccine development.
A Dutch court has ruled that billionaire and global vaccine proponent Bill Gates will face trial in the Netherlands over his involvement in misleading the public about the safety of COVID-19 vaccines, The Defender reported. The case, brought forward by seven plaintiffs who claim to have suffered vaccine injuries, marks a significant blow to Gates, who has been a key figure in pushing COVID-19 vaccination efforts worldwide.
According to Dutch newspaper De Telegraaf, the plaintiffs filed the lawsuit last year, naming Gates as one of 17 defendants, along with former Dutch Prime Minister and current NATO Secretary General Mark Rutte, members of the Dutch government’s COVID-19 Outbreak Management Team, Pfizer CEO Albert Bourla, and the Dutch state itself. The Bill & Melinda Gates Foundation has become one of the World Health Organization’s (WHO) most significant donors in recent years, contributing hundreds of millions of dollars annually. They have also been reported to have been kicked out of many countries for crimes against humanity. One such country is India, where the Bill & Melinda Gates Foundation and their vaccine empire are under fire, including a pending lawsuit currently being investigated by the India Supreme Court. They are also accused of spending hundreds of millions of dollars in grants to control social media fact checkers and news outlets. As of June of 2020, Bill Gates has given no less than $250 million in foundation grants into mainstream journalism.
Watch video 1
Dr. Carrie Madej has lost her license to practice medicine and her research has been heavily redacted, shadowbanned and discredited. She also has been harassed, had her life threatened and still continues to put out information that is disturbing and are crimes against humanity if true. But of all the accusations this foundation has against it, is it not far fetched to think they have an agenda, which could be depopulation and are simply carrying out what Bill said in public? He said in a TedTalk that he will use Vaccines to lower the population levels. That is a fact. And is it responsible to experiment on an unexpecting and unwilling population or simply put without their will? After all, a top Nigerian official has accused Microsoft co-founder Bill Gates of using the people of Africa as guinea pigs for his scientific experiments. And doctors in Kenya have accused UNICEF, the World Health Organization and the Bill and Melinda Gates Foundation of secretly trying to sterilize millions of women in Africa via a tetanus vaccine program.
Watch video 2
It seems irresponsible to say such things especially when Lipid nanoparticles are still brand new technology. The dangers that follow mRNA gene therapy drugs and the idea that the nanoparticles can hold deadly diseases for a later release is frightening. It’s easy to just accuse without having sufficient evidence to support your facts, but sometimes, you can just read between the lines. I did some digging on Lipid nanoparticles and found out they are more dangerous than we could ever imagine.
Lipid nanoparticles (LNPs) are a key component of many modern drug delivery systems, particularly in mRNA-based vaccines like those developed for COVID-19. While LNPs have revolutionized medicine by enabling the efficient delivery of genetic material into cells, they are not without potential risks and dangers. One of the primary concerns with LNPs is their potential to trigger immune responses. LNPs can activate the immune system, leading to inflammation or allergic reactions. This is particularly concerning for individuals with pre-existing autoimmune conditions or heightened immune sensitivity. Symptoms may include localized inflammation at the injection site, systemic inflammatory responses such as fever or fatigue, and, in rare cases, severe allergic reactions like anaphylaxis.
Another significant concern is the potential for toxicity and organ damage. LNPs can accumulate in certain organs, such as the liver, spleen, and lungs, due to their small size and the body’s natural filtration systems. This accumulation raises concerns about potential toxicity and long-term damage to these organs. For example, the liver, being a primary site for LNP accumulation, could experience inflammation or dysfunction. Similarly, if LNPs reach the lungs, they could cause inflammation or other respiratory issues. These risks highlight the need for careful monitoring of individuals receiving LNP-based treatments.
Off-target effects are another potential danger associated with LNPs. While they are designed to deliver their payload, such as mRNA, to specific cells, they can sometimes end up in unintended tissues or organs. This off-target delivery can lead to unintended effects, such as the production of proteins in the wrong cells, potentially disrupting normal cellular functions, or the activation of immune responses in unintended areas, leading to localized inflammation or damage. These off-target effects underscore the complexity of using LNPs in medical treatments.
The long-term effects of LNPs on the human body are not yet fully understood, as they are a relatively new technology. Questions remain about how long LNPs persist in the body, whether they can cause chronic inflammation or autoimmune disorders, and their potential effects on reproductive health or fetal development. These uncertainties highlight the need for ongoing research to fully understand the implications of LNP use in medicine.
There have also been reports of rare but serious cardiovascular side effects associated with LNP-based vaccines. These include myocarditis and pericarditis, which are inflammations of the heart muscle or lining, particularly observed in young males. Additionally, some studies suggest that LNPs could interact with blood components, potentially increasing the risk of clotting disorders. These cardiovascular risks are an important consideration, especially for individuals with pre-existing heart conditions.
LNPs can interact with the immune system in complex ways, potentially leading to overactivation of immune responses, causing cytokine storms or other systemic reactions. In some cases, they might also cause immune suppression, which could increase susceptibility to infections. These interactions highlight the delicate balance required when using LNPs in medical treatments to avoid unintended immune system consequences.
Beyond individual health risks, there are broader concerns about the use of LNPs, including their environmental impact and ethical issues. The long-term environmental effects of LNPs, particularly if they enter water systems or ecosystems, are not well studied. Additionally, the use of LNPs in experimental treatments or vaccines raises ethical questions about informed consent and the potential for unequal distribution of risks and benefits. These concerns emphasize the need for comprehensive studies and ethical considerations in the development and deployment of LNP-based technologies.
Finally, the production of LNPs requires precise control over their size, composition, and stability. Variations in manufacturing processes could lead to inconsistent performance of the drug or vaccine and increase the risk of adverse effects due to impurities or improper formulation. Ensuring high-quality manufacturing standards is crucial to minimize these risks and ensure the safety and efficacy of LNP-based treatments.
In conclusion, while lipid nanoparticles have enabled groundbreaking advancements in medicine, particularly in mRNA vaccines and gene therapies, they are not without risks. The potential dangers include immune reactions, organ toxicity, off-target effects, and unknown long-term consequences. Ongoing research is essential to better understand these risks and develop strategies to mitigate them. For now, the benefits of LNPs in treating diseases like COVID-19 are considered to outweigh the risks for most people, but continued monitoring and transparency are crucial to ensure their safe and effective use.
Without the lipid nanoparticles, mRNA cannot enter human cells, and the body would recognize the mRNA as a foreign and destroy it. In order to allow the mRNA to travel throughout the body, the mRNA is encased in lipid nanoparticles.
The lipid nanoparticles are synthetic fat molecules that mimic natural fat molecules. This means that they are not recognized by the body as a threat and are not destroyed by immune response as a foreign invader, which allows them to enter and release their mRNA cargo inside of cells. The mRNA then takes over and tricks the cells into synthesizing its foreign protein.
Lipid nanoparticles (LNPs) are synthetic lipids developed in the laboratory, and the technology has many unknowns. The technology is so new that there are no long-term studies on their health impacts. However, studies done before Pfizer was granted Emergency Use Authorization (EUA) revealed LNPs travel everywhere in the body and collect in most, if not all, organs. Pfizer told the world its mRNA gene therapy drug stays in the arm into which it is injected, even though the Pharma giant knew from its own biodistribution study that that was a lie. [“A Tissue Distribution Study of a [3H]-Labelled Lipid Nanoparticle-mRNA Formulation Containing ALC-0315 and ALC-0159 Following Intramuscular Administration in Wistar Han Rats,”
In nature, nanoparticles have always existed. Carbon nanotubes, discovered in the coating of pottery from India dating 600-300 BC, enabled it to last for centuries. However, there is no way to know if the nanotubes were there by accident or not. Also found circa 900 AD, Damascus steel contained cementite nanowires, and the origins and how they were made are unknown.
In the eighth-century Mesopotamia, which is modern-day Iraq, artisans were known for creating a glittering effect on a pot’s surface known as Lusterware. The process involved applying a mixture that included metal nanoparticles applied on previously glazed items, giving it an iridescent, metallic luster.
Modern-day nanotechnology first began in 1833 when the word “polymer” was introduced by a world-renowned Swedish chemist, Jöns Jakob Berzelius.
A year later, he introduced the term “isomer,” meaning substances with identical but differing properties. In other words, the substances have the same number and types of atoms but differ because the arrangement of the atoms makes them have different chemical structures.
This later helped launch an explosion of new synthetic polymers in the 1930s and 1940s, especially after World War II, including polyvinyl chloride (PVC), polyurethane (PU), nylon fibers, neoprene (synthetic rubber), polytetrafluoroethylene (PTFE or Teflon), and polystyrene (PS). Suddenly, synthetic fibers, films, plastics, rubbers, coatings, and adhesives were everywhere.
But these petroleum-sourced polymers contrast with biopolymers. Biopolymers are polymers from sources in nature and can be produced chemically from biological materials or are made by living organisms.
In 1965, Alec D. Bangham, a British biophysicist discovered liposomes (derived from two Greek words: lipo (“fat”) and soma (“body”)), and composed primarily of phospholipids, which are phosphorus-containing complex lipids and closed lipid bi-layer vesicles.
Bangham found that liposomes could be made spontaneously in water. These were the earliest version of lipid nanoparticles. Liposomes became the most widely studied and recognized drug delivery platform due to their biocompatibility and biodegradability, which resulted in minimal adverse reactions. They are currently used in skin care products and in nutritional supplements as well.
Liposomes used for drug treatments have had challenges, including poor stability and storage, and they are affected by pH and temperature. They have a low encapsulation rate due to a leakage problem with water-soluble drugs. Now, the use of PEGylated liposomes helps to improve encapsulation efficiencies and the size of particles, giving them a “stealth” effect, which increases their circulation time in the body.
Liposomes are hollow with one or more rings of lipid bilayers and are spherical vesicles surrounding a watery core, used as a drug carrier, made mainly from phospholipids and other physiologic lipids.
LNPs are made with lipid, or fat, components other than phospholipids. They are novel synthetic and semi-synthetic lipids that have greater stability because reactants act very slowly. LNPs have a more rigid form and structure, and this gives them significant advantages over liposomes.
The term ‘lipid nanoparticle’ originated in the 1990s. Big Pharma’s seems to have its sights set on revolutionizing healthcare using LNPs. LNPs are tiny fat balls, measured in nanometers, at a size of less than 200nm. A nanometer is one billionth of a meter. In comparison, the width of a human hair is 80,000nm to 100,000nm.
The science behind LNPs is an idea taken from nature. They are created using a micelle-like structure that holds drug molecules in a non-watery core surrounded and protected by a double-layer membrane of different lipids. With gene therapy, it is the encapsulated artificial pseudouridine (Pfizer’s mRNA) carried into the cells, where it gets deposited and used to make spike protein.
This process of artificially introducing nucleic acids (DNA or RNA) into cells is only possible by through LNPs’ ability to mimic natural and, thus, familiar fat molecules. This mimicry gives LNPs the ability to increase biodistribution throughout the body and, therefore, circulate longer in the body without detection by the immune system. This is made possible by the development of lipid- and polymer-based carriers called PEGylated phospholipids, or polyethylene glycol (PEG) polymer, a hydrogel that coats the lipid nanoparticle and helps it imitate the biological membrane. Without it, the immune system would quickly destroy the mRNA as the enemy invader it is. LNPs are a danger because they hijack cells and cause them to release the encapsulated, artificial pseudouridine they carry into the cells so that a foreign spike protein will be produced.
COMIRNATY® is the FDA-approved version of BNT162b2, Pfizer’s EUA COVID “vaccine” product. Although not available in the United States, it is being presented interchangeably by the Food and Drug Administration (FDA) and Pfizer, with the only difference being in one of the liquid buffers within the solution. The LNP formulation is identical and referred to as PF-07305885 (BNT162b2) and lipids.
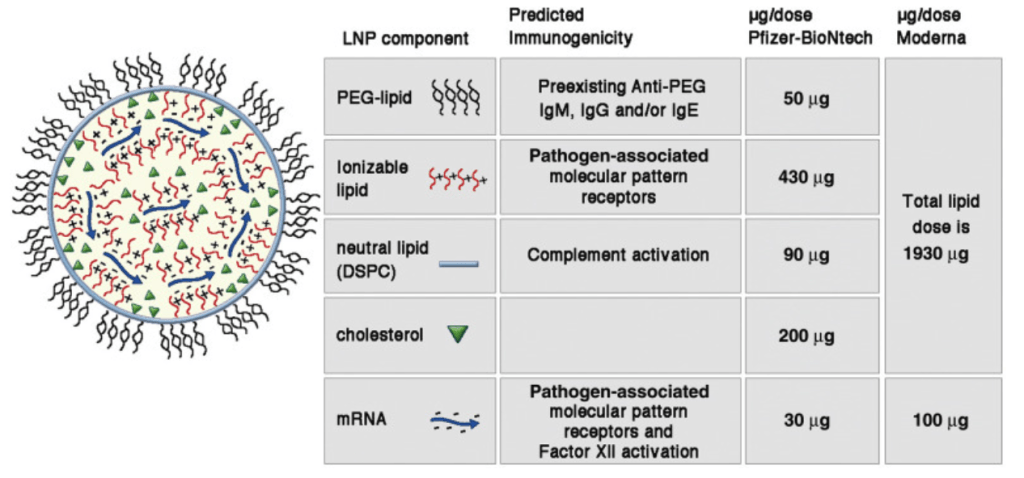
The Pfizer safety data and hazard sheet gives very limited information. Here are Pfizer’s stated components, along with the information this author found for each, as well as the warnings found attached to several of the components:
STRUCTURAL LIPIDS
ALC-0315 – ((4-hydroxybutyl) azanediyl) bis(hexane-6,1-diyl) bis(2-hexyldecanoate), a novel synthetic lipid, “proprietary” to Acuitas Therapeutics, and used in combination with other lipids to form lipid nanoparticles. A phospholipid makes up the basic structure of the nanoparticle wall, molecules modeled after phospholipids found in living cells. It is an ionizable, cationic lipid whose positive charge binds to negatively charged mRNA. It is positively charged at an acidic pH but neutral in blood. The pH-sensitivity is beneficial for delivery of the mRNA into the cells because neutral lipids have less interactions with the negatively charged membranes of blood cells, thus making it more biocompatible.
ALC-0159 – 2- [(polyethylene glycol)-2000]-N, N-ditetradecylacetamide – the second novel, synthetic lipid proprietary to Acuitas Therapeutics. It is a PEG/lipid conjugate (i.e., PEGylated lipid), PEG hydrogel, and it provides a hydrating layer surrounding the nanoparticle. It is functional and helps control the particle life and size, provides a hydrating layer which increases stability, helps prevent aggregation (clumping), and prolongs the circulation within the body.
DCPC – 1,2-Distearoyl-sn-glcerc-3-phosphocholine – a (semi) synthetic phosphatidylcholine that is a structural neutral lipid used as a component of the outer wall of the LNP. It has a cylindrical shape that allows its molecules to form a layered phase, stabilizing the structure of lipid nanoparticles.
Cholesterol – gives the LNP a polymorphic shape which helps to get the messenger RNA (mRNA) into the cells. It also aids fluidity of a lipid membrane depending on temperature, enables the encapsulated ingredients to be transported by the blood, and is a major constituent within the LNP. As a helper fat, it improves cellular delivery and stability, as well as contributing to the structure of the LNP.
Naturally-occurring cholesterol analogues in lipid nanoparticles induce polymorphic shape and enhance intracellular delivery of mRNA.
mRNA (messenger RNA) – has many different names stated by Pfizer in the safety sheet: Tozinameran is the generic name for BNT162b2, and the name COMIRNATY® is also being used. Additionally, it is listed as PF-07302048 Containing PF-07305885 (BNT162b2); CorVAC Containing PF-07305885 (BNT162b2); CoVVAC Containing PF-07305885 (BNT162b2); COVID Vaccine Containing PF-07305885 (BNT162b2); COVID-19 Vaccine Containing PF-07305885 (BNT162b2); BNT162b2 (BioNTech code number BNT162; and Pfizer code number PF-07302048).
Messenger RNA is a synthetically generated version of the coronavirus spike protein, changed to make it more bioavailable by introducing analogs to the RNA sequence grown in E-Coli bacterium. “The resulting mRNA is known as nucleoside changed RNA (modRNA); Full length SARS-CoV-2 spike protein bearing mutations preserving neutralization-sensitive sites.
(PF-07305885) – A mystery ingredient that is listed separately and named in the Pfizer safety sheet.
The mRNA itself is enveloped by ALC–0315 and cholesterol within the LNP.
See image 3
Pfizer did not disclose that tetrahydrofuran, a suspected carcinogen, is a solvent used in the manufacturing of the ALC-0159. There are other ingredients that were used in manufacturing this drug but that not disclosed to the public, because disclosure would allegedly expose a trade secret. In some cases, ingredients, such as processing aids, are used without data showing how much was allowed in the finished product. Examples include ethanol; citrate buffer; HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid), a zwitterionic sulfonic acid buffering agent; EDTA; residuals; metal substances or compounds; and by-products. Additionally, the public has not been told what quality control standards were applied or who was regulating the manufacturing process from start to finish.
There have been many requests for this information, but governments, including the United Kingdom, have denied Freedom of Information (FOI) requests under “Absolute and Qualified Exceptions.” As well as statements such as, “We do not give away commercial secrets concerning the manufacture and control of any medicinal product or its ingredients. The levels of ingredients, impurities and degradants are controlled in all authorized medicinal products so that they are safe.” So, the public has been given no real answer, as well as no transparency.
There are four salts in the Pfizer mRNA vaccine used to keep its pH consistent over time. Two of these salts, monobasic potassium phosphate and dibasic sodium phosphate dihydrate, are also used in some pharmaceutical treatments, as well as in fertilizers and as food additives. Potassium chloride and sodium chloride (i.e., table salt) are common, naturally occurring substances.
- Potassium Chloride – a buffering agent used to prevent a solution from becoming too acidic or basic.
- Disodium phosphate dihydrate – a buffer used to prevent a solution from becoming too acidic or basic.
- Sodium Chloride – a buffering agent used to prevent a solution from becoming too acidic or basic.
- Potassium Phosphate – a buffering agent used to prevent a solution from becoming too acidic or basic.
OTHER INGREDIENTS
- Deionized Water – neutral fluid to mix and dissolve ingredients.
- Sucrose – helps to stabilize ingredients, particularly at low temperatures.
- Tromethamine – “Tromethamine (a.k.a., “Tris”) is a blood acid reducer which is used to stabilize people with heart attacks.” This addition was found buried on page 14 in the Pfizer paperwork submitted to the FDA.
- Trometamol hydrochloride– an amine compound for pH control of metabolic acidosis.
The following two ingredients were discovered by a United Kingdom (U.K.) physician’s Freedom of Information (FOI) request to the U.K. government which requested disclosure of all of the vaccine ingredients. Only two ingredients were disclosed with a statement that all other information is protected and would not be shared. Both “added to the list of excipients in Section 6.1 of the SmPC for Pfizer’s experimental drug for the purpose of pH-adjustment. Neither is listed in Table 2 of Pfizer’s Summary Basis for Regulatory Action (SBRA).”
Sodium Hydroxide– This is lye, a highly caustic and reactive inorganic base.
Hydrochloric Acid – A water-based, or aqueous, solution of hydrogen chloride gas, also known as muriatic acid; a very strong acid.
Pfizer encapsulates the mRNA inside the LNP through synthetic production methods, a process that runs a solution of the lipids alongside another solution containing the mRNA in a buffer, such as acetic acid, and then puts them through a microfluidic mixer. These two solutions are close to each other as they are run through the mixer and spontaneously and rapidly combine forming LNP with mRNA encased inside it.
They get bottled and need to be stored in ultra-low temperature freezers from -60°C to -80°C (or -76°F to -112°F).
Chilling and avoiding sunlight or ultraviolet (UV) light as well as shaking the vial helps to slow down chemical reactions called oxidation. Oxidation degrades the LNP and will cause leakage and clumping of the lipids. The instructions state the LNP/mRNA vials need to thaw before use. Vials may be stored at -25ºC to -15ºC (-13ºF to 5ºF) for only up to two weeks.
Dr. Chris Flowers did a report on quality control of the vaccines in which he states:
“Quality control is a major issue given that mRNA is very unstable, reported by the European Medicines Agency and published in the British Medical Journal (BMJ) , and the LNP platform is tricky to get right consistently, both for the size of the particles and the distribution of mRNA within them.
Furthermore, there are technical issues with the mRNA/LNP platform which require ultra-low temperature freezers to maintain the integrity of these lipid particles, as they are subject to oxidative degradation where the lipids form into clumps. Indeed, there are many issues with the LNP storage and transport, as their integrity can be easily destroyed by vigorous shaking, including using road transport.” (https://dailyclout.io/failure-of-serialization-by-pfizer-flouted-established-pharma-rules/)
Pfizer’s protocol calls for the vial contents to be inspected before use and that they should appear as a white to off-white suspension that may contain white to off-white, opaque, shapeless particles. If it appears discolored or has other particles in it, then it is to be discarded. Depending on the formulation, a saline dilution may need to be added before use. Then, the vial should be gently inverted 10 times, not shaken, and discarded after six hours.
Changes in the LNP/mRNA appearance are from chemical reactions in the drug compound. As it degrades, one or more substances can be changed into something else. There is a loss of electrons, which always occurs accompanied by oxidative reduction and is also the reason drugs have a limited shelf life.
Whether from storage, handling, and administration protocol errors or not, chemical transformation happens. However, it will happen faster if the protocol is not followed properly. Sub-zero freezing, refrigeration, limited use time, no sunlight or UV light exposure, and no shaking are all stipulated, which helps slow down the chemical reactions. However, it is a challenging protocol to adhere to in all instances. If the LNPs break down, they leak their mRNA gene therapy drug cargo, so less of the mRNA gets into the cells.
In their administrative factsheet, Pfizer mentions the possibility of seeing “other particles” in the vials. Why would there be “other particles?” Could it be it from degradation, and/or are its other ingredients not disclosed, or both? Scientists, doctors, and other researchers have analyzed and discovered other elements in COVID-19 drug vials — ingredients that Pfizer and other companies have not published, disclosed, explained, or categorically denied the existence of in their formulations. Black specks and a metallic-like substance were seen in these vials, and a group of independent German scientists found toxic components that are metallic elements in AstraZeneca, Pfizer, and Moderna vaccine vials.
These scientists claim their results have been cross confirmed using the following measuring techniques: “Scanning Electron Microscopy, Energy Dispersive X-ray Spectroscopy, Mass Spectroscopy, Inductively Coupled Plasma Analysis, Bright Field Microscopy, Dark Field Microscopy and Live Blood Image Diagnostics, as well as analysis of images using Artificial Intelligence.”
Their findings are as follows:
See image 4

The German scientists also did a blood analysis on vaccinated and unvaccinated people and provided pictures of their findings, which they also submitted to the German government authorities for review. Many others have done analyses on the vial contents with similar findings.
Hundreds of doctors, scientists, and other professionals from over 34 countries signed and presented a manifesto declaring an international medical crisis caused by the COVID-19 LNP mRNA injections.
The medical crisis declaration states:
“A large number of adverse side effects, including hospitalizations, permanent disabilities and deaths related to the so-called ‘COVID-19 vaccines’, have been reported officially. The registered number has no precedent in world vaccination history. “
Examining the reports on CDC’s VAERS, the UK’s Yellow Card System, the Australian Adverse Event Monitoring System, Europe’s EudraVigilance System and the WHO’s VigiAccess Database, to date there have been more than 11 million reports of adverse effects and more than 70,000 deaths co-related to the inoculation of the products known as ‘covid vaccines’.
We know that these numbers just about represent between 1% and 10% of all real events. Therefore, we consider that we are facing a serious international medical crisis, which must be accepted and treated as critical by all states, health institutions and medical personnel worldwide.”
The absolute critical change demands a worldwide stop to all mRNA countermeasures.
NANOPARTICLE INVASION
Nanotechnology and nanoparticles are now used in electronics, healthcare, chemicals, cosmetics, composites, and energy. Nanoparticles are exceedingly small, from 1nm to 100nm, in size and are not visible by the human eye. Their movement cannot be controlled, and there is an inability to predict results for the outcome of changing molecules, which may mean undesirable results.
There can be significant health issues, such as malignant tumors, for those handling nanoparticles, because they can enter the body through the skin or inhalation. Once in the body, it is difficult, if not impossible, to get them out.
Lipid nanoparticles have had failures and toxicity issues in the past, so novel lipids were formulated to withstand the body’s defenses, thus allowing the LNPs to get their cargo into the cells. Dr. Richard Urso is a scientist, inventor, and medical doctor in practice since 1988. He was the former Chief of Orbital Oncology at the University of Texas MD Anderson Cancer Center and is a drug designer, a treatment specialist, and the sole inventor of an FDA-approved wound-healing drug. He studied and used LNPs in chemotherapy treatments. But he has been against the use of these LNP/mRNA drugs.
He discovered the dangers of the LNP delivery platform from their use in his patients’ chemotherapy treatments and abandoned them because of similar serious harms now arising from COVID-19 LNP/mRNA gene therapy drug treatments.
In a 2022 interview, Dr. Urso said, “From [ages] 25 to 44, we saw last quarter of last year an 82% rise in deaths, so there’s a lot of data that’s out there that is very, very troubling… This lipid nanoparticle messenger RNA platform, I don’t care what you attach it to, it is always going to travel everywhere. It’s always going to be a problem. And that’s why you see the distribution of disorders coming from this after the vaccines affect so many different organ systems because it distributes everywhere.”
LNPs travel throughout the body and accumulate in organs and the brain. Wherever mRNA encased in LNPs goes, it also delivers spike protein . [“A Tissue Distribution Study of a [3H]-Labelled Lipid Nanoparticle-mRNA Formulation Containing ALC-0315 and ALC-0159 Following Intramuscular Administration in Wistar Han Rats,”
Dr. Urso also said, “…the mRNA stays in the body for up to 15 months because the body is unable to degrade it and is not sure what to do with it, so it causes ongoing inflammation and a disruption of the immune system. The spike protein is a huge disrupter of blood products as well, the evidence shows this LNP platform for this gene therapy is terrible in many ways because it is distributed in every part of the body with no way to control it, creating inflammation in multiple organ systems throughout the body and has caused more deaths and injuries than all the vaccines combined over the last 30 years.”
Dr. Urso is the co-founder of the Global Covid Summit, an international alliance of over 17,000 physicians and medical scientists who put out a declaration stating:
- The state of medical emergency must end
- Scientific integrity must be restored
- COVID-19 injections must stop
- Crimes against humanity must be addressed.
Dr. Russell L. Blaylock also spoke out against the COVID experimental LNP/mRNA drugs. He, a prominent neurosurgeon now retired, was a clinical assistant professor of neurosurgery at the University of Mississippi Medical Center and now is a visiting professor in biology at Belhaven University, as well as a published author. He wrote a hard-hitting article in the National Library of Medicine about the lies and corruption surrounding the COVID-19 “pandemic.”
Dr. Blaylock confirms the biodistribution of the LNPs. He wrote, “The Japanese resorted to a FOIA (Freedom of Information Act) lawsuit to force Pfizer to release its secret biodistribution study. The reason Pfizer wanted it kept secret is that it demonstrated that Pfizer lied to the public and the regulatory agencies about the fate of the injected vaccine contents (the mRNA enclosed nano-lipid carrier). They claimed that it remained at the site of the injection (the shoulder), when in fact their own study found that it rapidly spread throughout the entire body by the bloodstream within 48 hours. The study also found that these deadly nano-lipid carriers collected in extremely high concentrations in several organs, including the reproductive organs of males and females, the heart, the liver, the bone marrow, and the spleen (a major immune organ). The highest concentration was in the ovaries and the bone marrow. These nano-lipid carriers also were deposited in the brain.”
The biodistribution study used luciferase enzyme, which produces a bioluminescent substance giving off light, and radioisotope markers to accurately track the distribution of LNPs from COVID mRNA drugs. It clearly showed LNPs went throughout the body.
Inflammation from LNPs can affect the vagus nerve, which controls the diaphragm and, thus, breathing. It is the tenth of the twelve cranial nerves and the longest, most important in the human body that directs the inner nerve center, the parasympathetic nervous system.
The vagus nerve originates in the brain stem and wanders down through the body branching off into multiple organs including the thymus, heart, lungs, liver, gallbladder, stomach, pancreas, kidneys, adrenals, spleen, intestines, ovaries, and testes. Seventy-five percent of its function involves sending information from the organs it controls to the brain, so it is the “mind-body connection.” A higher vagal tone is associated with better overall health and well-being. The sensory signals from internal organs travel through the vagus nerve to the basal ganglia, which is a part of structures deep within the brain, and it enables the brain to keep track of organ actions. It helps regulate functions like digestion, heart rate, blood pressure, vascular tone, respiration and even speaking. Moreover, it also helps control reflex actions like coughing, swallowing, gagging, and sneezing.
Damage or dysfunction to the vagus nerve can affect one’s mood, digestion, cardiovascular system and has been linked to on-going inflammation, leading to chronic diseases, cancer, cardiovascular issues, neurodegeneration, and even death. Interference with the vagus nerve can cause partial or completely blocked electrical impulses in the heart. Depending on the severity of damage to the vagus nerve along with other health conditions, the symptoms can range from none to dizziness, fainting, or death.
LNPs are supposedly safe and non-toxic, but mice studies done with intradermal injection of LNPs caused inflammation and showed the fast movement, scattering, and distribution rate of LNPs into tissues. The intranasal inoculation caused approximately 80% of the mice to die in less than 24 hours. So, LNPs were known to be highly inflammatory before Pfizer’s human trials started.
Now, since LNP/mRNA drugs have been given to humans, inflammation is occurring throughout people’s bodies and weakening their immune systems. The inflammation is causing injury and/or further damage to weak or diseased systems and organs. Since COVID LNP/mRNA injections began, there have been significant increases in many serious, sudden, and even fatal heart conditions; increases in cancers; neurological problems; circulatory problems; and more . Each additional shot taken can increase the number and severity of adverse effects.
The FDA was taken to court to stop them from keeping Pfizer’s clinical trial documents secret. A Texas court ordered the FDA to release 450,000 pages of documents starting on January 31, 2022. Without access to these documents, patients were not able to give informed consent, because the drug-related risks were not fully disclosed.
Pfizer had the largest healthcare fraud settlement in the history of the Department of Justice on September 2, 2009. Pfizer was fined $2.3 billion for fraudulent marketing.
Now Pfizer is making billions by manufacturing and marketing a completely ineffective “vaccine.” Deepak Kaushal, who admitted to faking data ten separate times with federal grant applications and had a paper retracted, is the co-author of the Pfizer COVID-19 vaccine animal study, by which Pfizer’s BNT162b2 was determined “safe and effective.” Why was a known perpetrator of fraud allowed to participate in the study that led to the FDA’s EUA approval for a treatment deployed to billions of people?
Insurance companies are reporting historically high rates of serious health issues and death from non-COVID-19-related causes since the LNP/mRNA shots rolled out to the public. Young people are experiencing myocarditis, heart attacks, strokes, and sudden deaths, none of which is normal for that demographic. Dr. Peter McCullough explained the findings out of Thailand showing one in 43 adolescents are having heart issues, including subclinical myocarditis, a silent killer. He said, as a cardiologist, “There is no heart damage that is mild or inconsequential, and all COVID vaccines should be stopped for all young people.
Embalmers have found unnatural, “whitish” clots in the arteries and vessels of those who have died after receiving mRNA COVID shots. Richard Hirschman, an embalmer, has been documenting these strange clots since 2021.
Lipid nanoparticles cause harms to humans, and there is no known way to remove them from the body. Because these harms — as well as the facts that LNPs travel throughout the body, lodge in organs, and cannot be removed — were not disclosed to patients before they received the LNP/mRNA COVID “vaccines,” patients were not able to provide informed consent to receiving the experimental COVID medications. The American Medical Association (AMA), which has aggressively pushed COVID vaccines, says, “Informed consent to medical treatment is fundamental in both ethics and law. Patients have the right to receive information and ask questions about recommended treatments so that they can make well-considered decisions about care…In seeking a patient’s informed consent…physicians should:
Assess the patient’s ability to understand relevant medical information and the implications of treatment alternatives and to make an independent, voluntary decision.
Present relevant information accurately and sensitively, in keeping with the patient’s preferences for receiving medical information. The physician should include information about:
The nature and purpose of recommended interventions
The burdens, risks, and expected benefits of all options, including forgoing treatment
Document the informed consent conversation and the patient’s…decision in the medical record in some manner. When the patient…has provided specific written consent, the consent form should be included in the record.”
Because these basic steps were not taken, governments, public health agencies, medical professionals, and corporations acted, according to the AMA, in an unethical manner toward billions of people who have received the COVID vaccines worldwide. Americans and others must take legal action against those who failed to complete one of the most basic tenets of ethical medical interventions.
source
Deepseek.com
